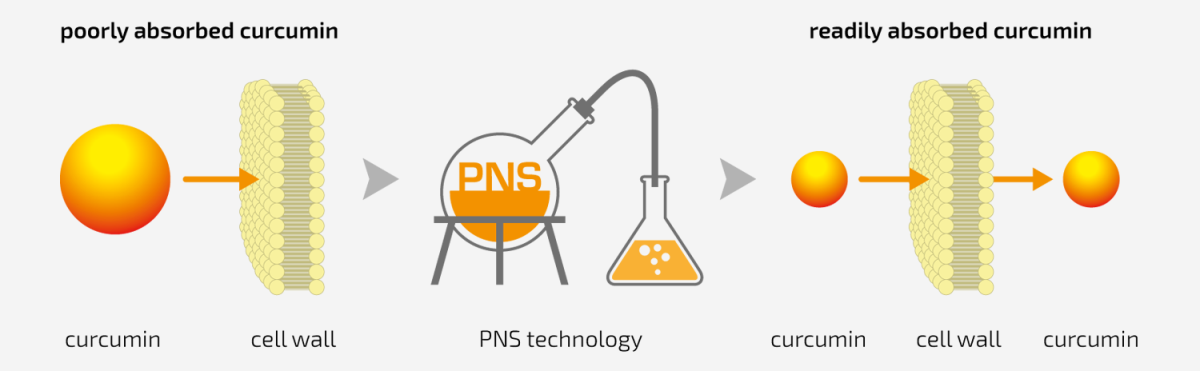
PNS technology
Smooth penetration of Curcumin into target tissues
Traditionally extracted Curcumin would not pass through the cell membrane easily, thus making it less bioavailable. Curcumin forms
large molecular aggregates, which leads to their poor absorption.
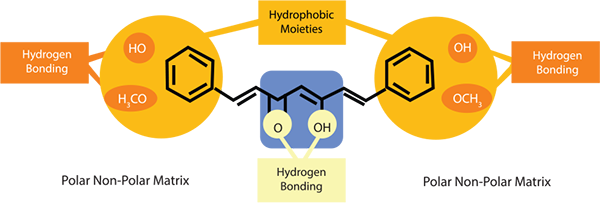
The PNS technology allows curcumin to be sandwiched
between the polar and non-polar molecules. This process of simple diffusion allows it to pass through the cell membrane easily.
The PNS technology has the advantage of not using any other
adjuvant or substrate (e.g. piperine) to increase the bioavailability.
As the product incorporates a complete turmeric matrix, it can boast two important features-high bioefficacy and cellular response. The synergic interaction of curcumin with other ingredients of turmeric makes it a highly bioefficient ingredient.



PNS technology
Increased bioavailability of Cureit, compared to the conventional curcumin formulations
The extraction process of Cureit is based on PNS Technology (Polar Non-polar Sandwich Technology), which contributes to the increased bioavailability of Cureit, compared to the conventional curcumin formulations.
For a molecule to be Bioavailable, it has to effectively reach the blood stream. When consumed orally, large molecule has to pass throught the inner intestinal wall to reach the blood stream. This involves moving through the cell wall which is predominantly composed of lipids, i.e. fat soluble or hydrophobic molecule can easily slip through.
Curcuminoids are fat soluble but because of their structure they tend to pass through the cell wall in the form of molecular aggregates, and these are poorly absorbed, so methods are sought to facilitate their penetration into the body.

PNS technology
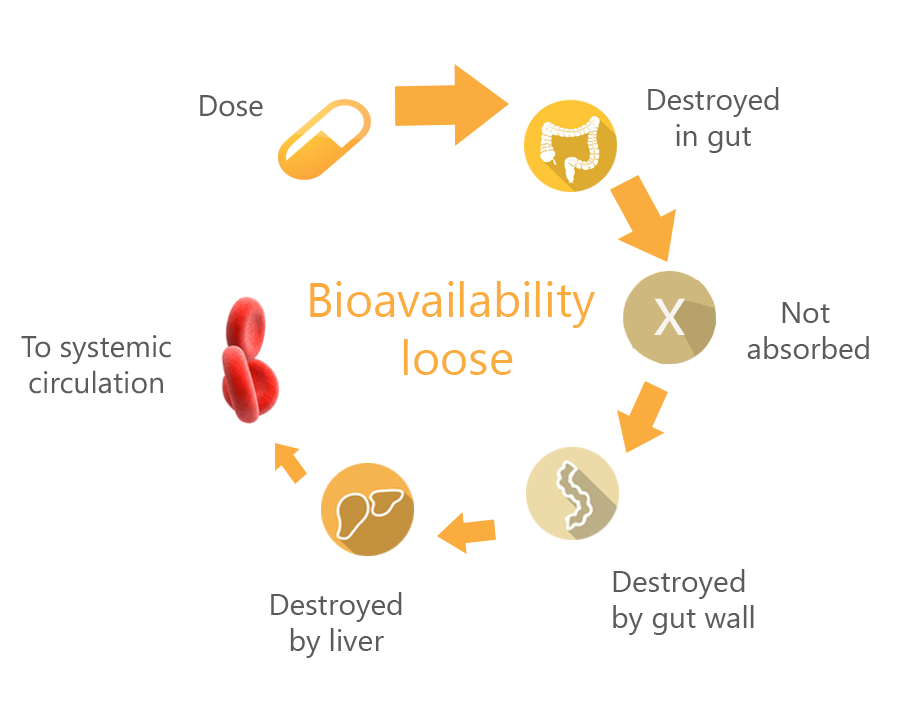
Factors that decrease oral bioavailability
Orally administered drugs must pass through the intestinal wall and then the portal circulation to the liver; both are common sites at first – pass metabolism (metabolism that occurs before a drug reaches systemic circulation). The efficacy of the orally administered substance is substantially reduced even before the substance enters the blood stream.
Thus, many drugs may be metabolized before adequate plasma concentrations are reached. Low bioavailability is most common with oral dosage forms of poorly water soluble, slowly absorbed drugs.

| Polarity | Turmeric content |
|---|---|
| Non polar molecules | Essential oils |
| Polar molecules | Curcumin |
| Non polar molecules | Carbohydrates, dietary fiber, protein |

PNS technology
Polar and Non-polar molecules
Raw tumeric was subject to three different extractions. These three components are put together to make the Polar and Non-polar soup. The bioactive molecule of curcumin is preserved inside this soup.
Owing to the combination nature of the hydrophilic and hydrophobic molecules, Cureit readily passes through the cell wall and thus can be easily absorbed. This unique PNS Technology raises the bioavailbility of Curelt over other conventional curcumin extracts.
Extraction by this PNS Technology also has the plus of not using any other adjuvant or substrate (e.g.: piperine) to increase the bioavailbility.
PNS Technology provides Cureit brand logo with the unique advantage of containing safely extracted curcumin with the increased bioavailability. PNS Technology is also supported by the evidence that links the increased bioavailablity of the Polar curcumin with the presence of Non polar components.